Inflammatory bowel disease (IBD) affects millions, disrupting daily life with symptoms like abdominal pain, diarrhea, and fatigue. As the demand for effective treatments grows, clinical trials offer a glimpse into promising new therapies. Trials play a vital role in advancing medical understanding and providing patients with access to cutting-edge care. By participating in or following the progress of IBD clinical trials, individuals can stay informed about potential breakthroughs that may transform treatment options.
Understanding Inflammatory Bowel Disease
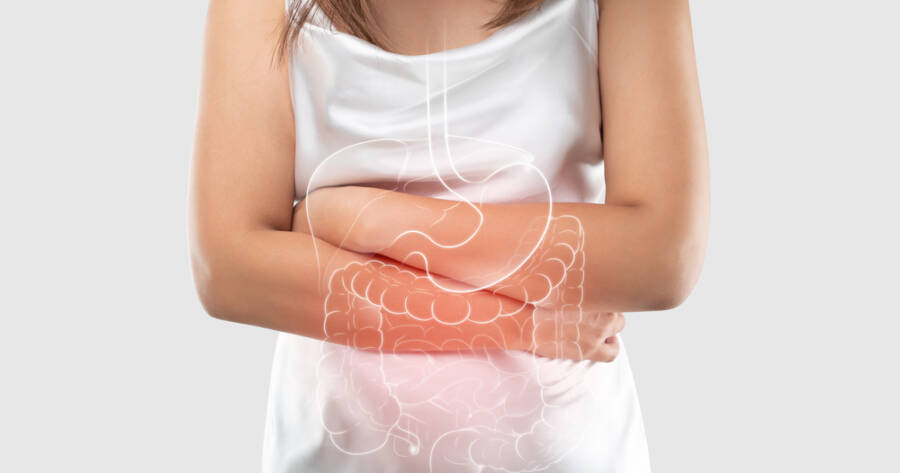
Inflammatory bowel disease includes chronic conditions such as Crohn’s disease and ulcerative colitis, causing persistent inflammation in the digestive tract. Symptoms can be debilitating, impacting nutrition, energy, and overall well-being. While the exact cause of IBD is unclear, it is believed to involve an abnormal immune response in genetically predisposed individuals. Current treatments typically focus on reducing inflammation and maintaining remission.
Despite the availability of medications, many patients face challenges in managing their symptoms. Side effects and variable efficacy often necessitate the development of alternative treatments. As researchers strive to better understand IBD’s complexities, clinical trials play a vital role in exploring potential therapies that address these needs.
IBD Clinical Trials
Finding and participating in clinical trials for inflammatory bowel disease (IBD) can provide access to cutting-edge treatments and contribute to advancing medical research. A primary resource for locating such trials is ClinicalTrials.gov, a comprehensive database maintained by the U.S. National Library of Medicine. By searching for “Inflammatory Bowel Disease” and filtering by location, such as the United States, individuals can find a list of ongoing studies, including details about eligibility criteria, study objectives, and contact information.
Before enrolling, it’s essential to consult with your healthcare provider to determine if a specific trial aligns with your medical needs and treatment goals. Participation typically involves an informed consent process, where researchers explain the study’s purpose, procedures, potential risks, and benefits. Additional resources, like the Crohn’s & Colitis Foundation’s Clinical Trials Community, offer guidance and support for those considering trial participation. Engaging in a clinical trial not only offers potential personal health benefits but also plays a crucial role in the development of new therapies for IBD.
The Value of Volunteer Participation
Volunteer involvement is indispensable in clinical trials, furthering the progression of medical research. By participating, individuals help researchers deduce the treatment’s impact on health outcomes, delivering data that could refine or revolutionize current care strategies. Without volunteer support, it would be challenging to achieve the comprehensive insights needed to move these treatments closer to clinical application.
Beyond financial compensation, participation provides individuals with an opportunity to contribute to meaningful research and gain exposure to cutting-edge medical science. Volunteers in this trial join efforts to enhance IBD management, potentially impacting future treatment landscapes. Sharing in this endeavor may offer satisfaction that extends beyond the individual, benefiting patients worldwide.
Emerging Trends in IBD Research
The emerging trials represent a broader trend in exploring novel treatment avenues for IBD. Researchers are investigating various pharmaceutical options, including biologics that target specific components of the immune system, and small molecules aimed at reducing inflammation at a cellular level. Precision medicine approaches look promising, as they may allow tailoring treatments based on a patient’s unique genetic and molecular makeup.
Furthermore, growing interest in gut microbiome manipulation—through probiotics or microbiota transplants—highlights the potential of altering gut flora to influence disease course. Combining these strategies with advanced drug delivery systems could optimize treatment efficacy and minimize side effects. As this research evolves, it could redefine approaches to IBD management, improving patient experiences and outcomes.
Supporting Steps Toward New Solutions
As clinical trials for IBD continue, collaboration between healthcare providers, researchers, and volunteers remains crucial. By fostering an environment of innovation and cooperation, these initiatives can lead to breakthroughs once considered out of reach. Advocacy for continued investment and participation in clinical research can accelerate the discovery of new therapies, offering brighter prospects for those battling IBD.
Education and awareness also play vital roles in broadening the understanding of clinical trials’ importance. Engaging with patient communities, care providers can simplify complex concepts, empowering patients to make informed decisions regarding participation. This collective effort aims to create lasting change, promoting better health and well-being for those living with inflammatory bowel diseases.
Learn More Today
Clinical trials exemplify the push for novel IBD treatments that could enhance lives. With valuable volunteer contributions, these studies pave the way for transformative therapies, reflecting hope for improved disease management.
Participation in research efforts not only advances science but also empowers individuals to be part of significant change. By seeking out information and considering involvement, you may play a pivotal role in shaping future healthcare solutions. Through collaboration and innovation, the path towards effective IBD treatment continues to unfold, offering optimism for a healthier tomorrow.